Research
Protein Biophysics

Shun-ichi Kidokoro
Professor

Tomonori Saotome
Assistant Professor

We are interested in understanding the structural dynamics and biophysical properties of proteins at the atomic scale. Specific topics include the spontaneous and rapid folding, strong binding to the target molecules, and highly selective catalytic-reaction of heat shock proteins and proteases. The structure and physical properties of proteins are evaluated by our highly sensitive calorimeters (DSC, ITC, and PPC) and spectroscopic methods (UV absorbance, CD, and fluorescence). The obtained information will be used for rational designs of mutant proteins with desired properties, such as stability at high temperature and superior activity towards target substrates. Mutant proteins are produced in a large scale using bacteria or yeast expression systems and further evaluated for their biochemical and biophysical properties. By repeating these processes, we have successfully engineered proteases with10-fold higher activity and/or 20 degrees higher thermal stability than the native counterpart. These investigations not only provide the basic understanding of protein dynamics, but also generate proteins useful for industrial applications.
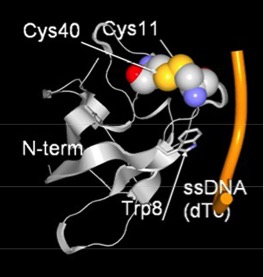
- Strong interaction between cold shock protein and single-stranded DNA
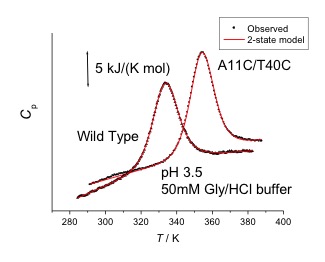
- Denaturation process of protease and its kinetic stability
- Rational design of highly stabilized proteins
- Hydration effect on ATP hydrolysis enthalpy